13.6 Ev
The binding energy of the hydrogen atom in its ground state is 13.6 eV. What is the energy when it is in the n = 5 state? StrategyThe amount of energy required to cause a transition from the ground state to the n= 4 state is equal to the difference in the energy between the two states. The energy for a hydrogen atom in the nth stationary state is given by En=(!13.6 eV)n2. SolutionThe energy of a hydrogen atom in the n= 4 state is 1 2. If potential energy of an electron in ground state is -13.6 eV. The energy of next higher state is: a) -27.2 eV b) -6.8 eV c) -3.4 eV d) none of these please help me. If I understand you question, yes, B is the correct answer. The energy of the levels are negative and go to zero when the electron is at infinity; i.e., when the atom is ionized. The NEXT high level would be C after B. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.
A54.4 eV
B6.8 eV
C13.6 eV
D24.5 eV
Solution:
For hydrogen atom Z = 1
$therefore, , , , Ionisation, energy, E_H=frac{2pi^2 me^4}{n_2 h_2}, , , , , , , , , , , ...(i)$
For $He^+ ion, (He^+ = 1s^1)$
so, $(He^+ = H)$ ionisation energy
$, , , , , , , , , , , E_{{He}^+}=frac{2pi^2 me^4 Z^2}{n^2 h^2}, , , , , , , , , , , , ...(ii)$
Eq (i)/Eq (ii), we get
$, , , , , , , , , , , , , , , , , E_{{He}^+}= E_H times Z^2$
$, , , , , , , , , , , , , , , , , , , , = 13.6 times 4$ = 54.4eV
1. Among acetic acid, phenol and n-hexanol which one of the following compound will react with $NaHCO_3$ solution to give sodium salt and $CO_2$ ?
2. If ionisation potential for hydrogen atom is 13.6 eV, then ionisation potential for $He^+$ will be
3. When chlorine is passed through propene at $400^{circ} C,$ which is the following is formed?
4. One of the characteristic properties of non-metals is that they
5. Which electronic configuration of an element has abnormally high difference between second and third ionisation energy?
6. Industrial preparation of chloroform employs acetone and
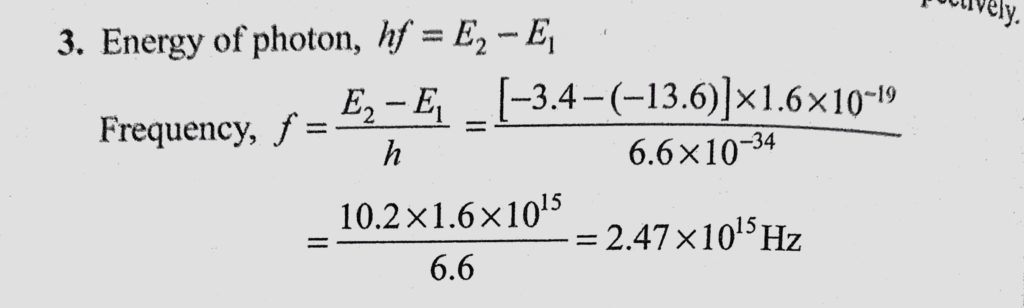
7. In the periodic table from left to right in a period, the atomic volume

8. If the atomic number of an element is 33, it will be placed in the periodic table in the
9. When hydrochloric acid gas is treated with
propene in presence of benzoyl peroxide, it
gives
10. What is formed when a primary alcohol
undergoes catalytic dehydrogenation?
1. The following nuclear transmission
$ce{^{23}_{11}Na + ^{1}_{1} H to ^{23}_{12} Mg + ^{1}_{0} n}$
belongs to
2. For emission line of atomic hydrogen from $n_i = 8$ to $n_f $ = the plot of wave number $(bar{v})$ against $( frac{1}{n^2})$ will be (The Ry dbergconstant, $R_H$ is in wave number unit).
3. Half-life of radium is 1580 years. Its average life will be
4. Which of the following arrangement is possible?
$quad$$quad$n$quad$ $quad$l$quad$$quad$m$quad$$quad$s
5. Quantum numbers of an atom can be defined on the basis of
6. Spectrum of Li2+ is similar to that of
13.6 Ev To Hz

7. Azimuthal quantum number defines
Hydrogen Energy Diagram
8. The quantum number m of a free gaseous atom is associated with
9. The isoelectronic pair is
10. For principle quantum number n = 4, the total number of orbitals having l = 3 is
1. In Wolff‐Kishner reduction, the carbonyl group of aldehydes and ketones is converted into
2. Identify compound X in the following sequence of reactions:
3. Identify a molecule which does not exist.
4. Identify the incorrect match.
Name IUPAC Official Name A Unnilunium i Mendelevium B Unniltrium ii Lawrencium C Unnilhexium iii Seaborgium D Unununnium iv Darmstadtium
| Name | IUPAC Official Name | ||
|---|---|---|---|
| A | Unnilunium | i | Mendelevium |
| B | Unniltrium | ii | Lawrencium |
| C | Unnilhexium | iii | Seaborgium |
| D | Unununnium | iv | Darmstadtium |
5. Reaction between acetone and methyl magnesium chloride followed by hydrolysis will give :
6. Identify the correct statements from the following:
(a) $CO_2(g)$ is used as refrigerant for ice-cream and frozen food.
(b) The structure of $C_{60}$ contains twelve six carbon rings and twenty five carbon rings.
(c) $ZSM-5$, a type of zeolite, is used to convert alcohols into gasoline.
(d) $CO$ is colorless and odourless gas.
13.6 Ev To Nm
7. Which of the following set of molecules will have zero dipole moment ?
8. On electrolysis of dil.sulphuric acid using Platinum (Pt) electrode, the product obtained at anode will be:
9. An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. The atomic radiusis:
10. Find out the solubility of $Ni(OH)_2$ in 0.1 M NaOH. Given that the ionic product of $Ni(OH)_2$ is $2 times 10^{-15}$
